The metabolism, CAncer and immune REsponses (mCARE) INSERM team, created in 2006, focuses on the molecular basis of energy metabolism in cancers (lymphomas and lung cancers).
Our research strategy is to determine whether modulation of cancer cell metabolism and metabolic enzymes can modulate tumor growth, response to chemotherapy and the anticancer immune response.

In cancer research, identifying specificities of tumor cells compared with "normal" proliferating cells is considered the holy grail for the development of targeted therapies. Although diverse in origin, most cancer cells share characteristics including the ability to escape cell death and the use of different sources of energy. In the current paradigm, aerobic glycolysis is considered the central metabolic characteristic of cancer cells (the Warburg effect). However, recent data indicate that significant changes in other metabolic pathways are also needed, including amino acid, lipid and mitochondrial (OxPhos) metabolism. In this more complex picture, this variety of metabolic source could be a strength (creating endless possibilities for adaptation and drug resistance) or a weakness that could be targeted to eliminate cancer cells.
Our laboratory studies the molecular basis of tumor metabolism in cancers: how it can be targeted and how it may influence the anticancer immune response – on a continuum from fundamental mechanisms to the clinic. We are developing unique preclinical models and in vivo methods to study the metabolic dependency of tumor cells in their correct pathological microenvironment.

Projects

JE. RicciResearch Director
Mail jean-ehrland.ricci@univ-cotedazur.fr

J. ChicheResearcher
Mail Johanna.CHICHE@univ-cotedazur.fr
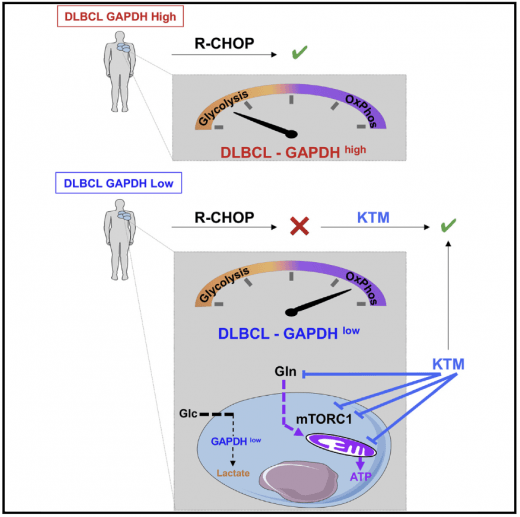
About 40% of diffuse large B cell lymphoma (DLBCL) patients are refractory to the combined immunochemotherapy (R-CHOP) standard treatment. DLBCL is metabolically heterogeneous. We have shown that low levels of GAPDH, which predict poor R-CHOP response, are associated with OxPhos metabolism, mTORC1 signaling, and glutaminolysis. Inhibitors of these pathways improved the response of DLBCL mice and patients with low levels of GAPDH. Chiche et al. Cell Metabolism 2019

R. Paul-BellonResearch engineer
Mail Rachel.PAUL@univ-cotedazur.fr

S. MarchettiResearcher
Mail Sandrine.MARCHETTI@univ-cotedazur.fr

A. AusselPhD student
Mail anais.aussel@etu.univ-cotedazur.fr

N. DoratResearch engineer
Mail noemie.dorat@sfr.fr

E. ChicheHead of University Clinic - Hospital Assistant (CCA)
Mail chiche.e@chu-nice.fr

N. MounierUniversity Professor - Hospital Practitioner (PU-PH)
Mail mounier.n@chu-nice.fr

L. ZawilPost-doctoral fellow
Mail leenazaweel@gmail.com

JE. RicciResearch Director
Mail jean-ehrland.ricci@univ-cotedazur.fr
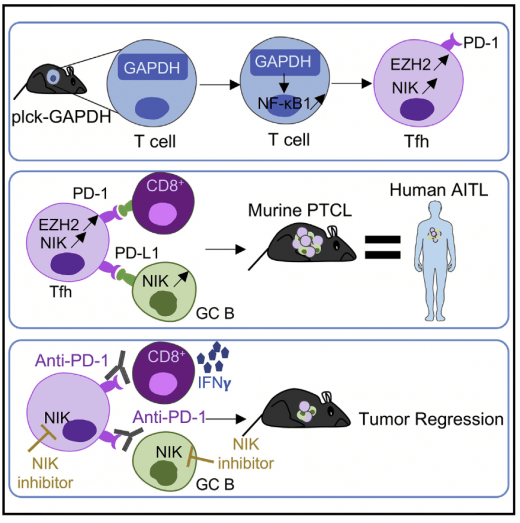
We generated transgenic mice overexpressing GAPDH in T cells. We obserevd that those mice develop lymphoma that recapitulates key features of human Angioimmunoblastic T-cell Lymphoma (AITL), including activation of the noncanonical NF-kB pathway. Blocking NF-kB activity with an NIK inhibitor reduces human and mouse AITL growth. Mondragon et al. Cancer Cell 2019

R. Paul-BellonResearch engineer
Mail Rachel.PAUL@univ-cotedazur.fr

JE. RicciResearch Director
Mail jean-ehrland.ricci@univ-cotedazur.fr
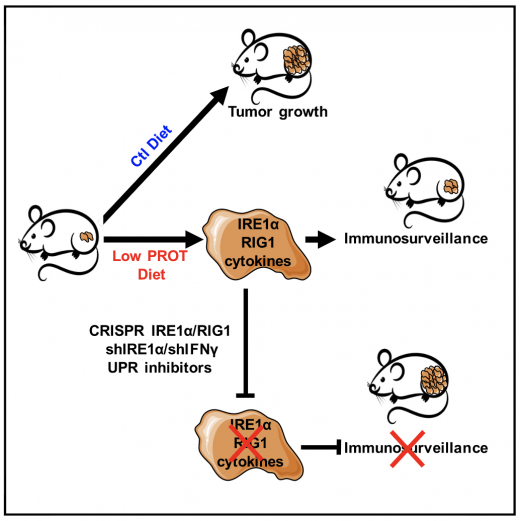
Dietary restriction (DR) slows down tumor growth by increasing tumor immunosurveillance. We showed that a moderate reduction in dietary protein intake, compared with a reduction in carbohydrates, without overall calorie changes, activates the IRE1a/RIG1pathway in tumor cells resulting in an anticancer immune response in mice. Rubio-Patino et al. Cell Metabolism 2018

H. IssaouiPost-doctoral fellow
Mail hussein_issaoui1992@hotmail.fr

R. Paul-BellonResearch engineer
Mail Rachel.PAUL@univ-cotedazur.fr

J. ChicheResearcher
Mail Johanna.CHICHE@univ-cotedazur.fr

S. MarchettiResearcher
Mail Sandrine.MARCHETTI@univ-cotedazur.fr

JE. RicciResearch Director
Mail jean-ehrland.ricci@univ-cotedazur.fr

S. MarchettiResearcher
Mail Sandrine.MARCHETTI@univ-cotedazur.fr
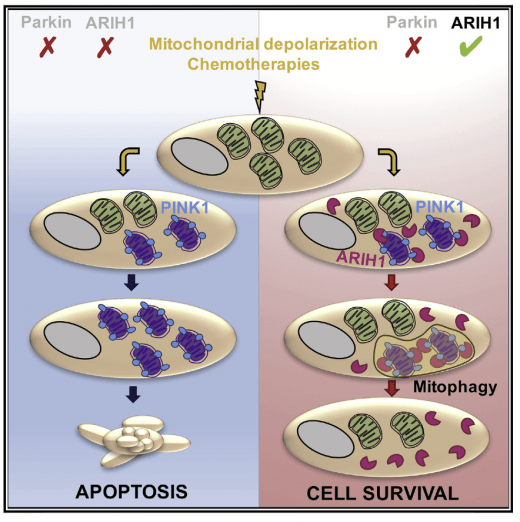
Clearance of damaged mitochondria (mitophagy) is involved in the resistance to chemotherapy-induced death, but the main known regulators of mitophagy are not expressed in cancer cells. We showed that the RBR E3 ligase ARIH1 is expressed in several cancer cell types. ARIH1 controls PINK1-dependent mitophagy and sensitivity to chemotherapies. Villa et al. Cell Reports 2017

S. Nunez-VasquezPost-doctoral fellow
Mail nunezvazquez.sonia@gmail.com

R. Paul-BellonResearch engineer
Mail Rachel.PAUL@univ-cotedazur.fr

J. ChicheResearcher
Mail Johanna.CHICHE@univ-cotedazur.fr

D. GoncalvesPhD student
Mail diogo.jumel-goncalves@etu.univ-cotedazur.fr

L. Di-MascioResearch engineer
Mail Lea.Di-Mascio@unice.fr
Publications
Focus
GAPDH Expression Predicts the Response to R-CHOP, the Tumor Metabolic Status, and the Response of DLBCL Patients to Metabolic Inhibitors.Authors Chiche J, Reverso-Meinietti J, Mouchotte A, Rubio-Patiño C, Mhaidly R, Villa E, Bossowski JP, Proics E, Grima-Reyes M, Paquet A, Fragaki K, Marchetti S, Briere J, Ambrosetti D, Michiels JF, Molina TJ, Copie-Bergman C, Lehmann-Che J, Peyrottes I, Peyrade F, de Kerviler E, Taillan B, Garnier G, Verhoeyen E, Paquis-Flucklinger V, Shintu L, Delwail V, Delpech-Debiais C, Delarue R, Bosly A, Petrella T, Brisou G, Nadel B, Barbry P, Mounier N, Thieblemont C, Ricci JE
Cell metabolism Jun 2019
Other recent publications
In vivo CAR T cell therapy against angioimmunoblastic T cell lymphoma.Authors Krug A, Saidane A, Martinello C, Fusil F, Michels A, Buchholz CJ, Ricci JE, Verhoeyen E
Journal of experimental & clinical cancer research : CR Sep 2024
Dependence on mitochondrial respiration of malignant T cells reveals a new therapeutic target for angioimmunoblastic T-cell lymphoma.Authors Krug A, Mhaidly R, Tosolini M, Mondragon L, Tari G, Turtos AM, Paul-Bellon R, Asnafi V, Marchetti S, Di Mascio L, Travert M, Bost F, Bachy E, Argüello RJ, Fournié JJ, Gaulard P, Lemonnier F, Ricci JE, Verhoeyen E
Cell death discovery Jun 2024
Bax Inhibitor-1 preserves pancreatic β-cell proteostasis by limiting proinsulin misfolding and programmed cell death.Authors Blanc M, Habbouche L, Xiao P, Lebeaupin C, Janona M, Vaillant N, Irondelle M, Gilleron J, Murcy F, Rousseau D, Luci C, Barouillet T, Marchetti S, Lacas-Gervais S, Yvan-Charvet L, Gual P, Cardozo AK, Bailly-Maitre B
Cell death & disease May 2024
Show all publicationsPatents
Co-inventors J. Chiche, J. Ricci, catherine Thieblemont
Co-inventors J. Chiche, J. Ricci, Catherine Thieblemont
Co-inventors J. Chiche, J. Ricci, Catherine Thieblemont


