Several PhD positions are proposed this year at C3M to start in autumn 2023.
These PhD positions are subject to obtaining a doctoral funding contract.
To apply for doctoral contracts, please contact the potential thesis director before 5 June 2023.
Team Fundamental to Translational Research on Dysregulated Hematopoiesis
Searching for new therapeutic strategies to treat the relapse of pediatric T cell acute lymphoblastic leukemia
Pediatric T-cell acute lymphoblastic leukemia (T-ALL) that comes from the transformation of T lymphocytes, is a highly aggressive form of cancer. Unfortunately, relapses occur in 10-15% of the cases and the prognosis is very poor, with almost 70% of children dying due to the lack of effective therapeutic options.
In this PhD project, we aim to gain a deeper understanding of pediatric T-ALL by dissecting the molecular and biological mechanisms responsible for resistance to chemotherapy. By identifying and characterizing the genes involved in resistance, we hope to discover new therapeutic targets, new active drugs as well as biomarkers to predict relapse.
To achieve these goals, we have used single-cell RNA sequencing technology to compare the gene expression profiles of pediatric T-ALL samples obtained at diagnosis or after relapse. This investigation at the level of individual leukemic cells highlights the heterogeneity of leukemic cells and their plasticity, tow essential properties supporting adaptation and resistance to chemotherapeutic drugs. Our bioinformatic analyses identified several candidate genes and signaling pathways that are potentially responsible for resistance to treatments, which we plan to validate during this thesis through functional assays in different cellular and mouse models.
Additionally, we have developed a T-ALL mouse KO model (tPTEN-/-) generated by the T-cell specific deletion of the PTEN tumor suppressor gene. Moreover, we generated double KO mice to study the role of the NF-kB transcription factor to orchestrate an anti-leukemic immune response. Modulating NF-kB activation in vivo either accelerates or delays the leukemic development. These different mouse models will be used to identify and characterize by flow cytometry and single-cell RNAseq, the different immune populations that participate in the anti-leukemic response.
Methods:
The candidate will benefit from several biological models and experimental approaches, including in vitro experiments on human immortalized cell lines and ex-vivo experiments on T-ALL patient-derived xenografts (PDX), established by the team in immunodeficient NSG mice. tPTEN-/- mice will be used to investigate in a pre-clinical context, the molecular mechanisms of leukemia development and to test new anti-leukemic drugs in vivo.
Flow cytometry will be routinely used for functional analyses (proliferation/survival/death). Diverse assays will monitor cell proliferation, mitochondrial activity (WST1), cell metabolism (glycolysis/Oxphos by SeaHorse). Signaling pathways will be investigated through Simple Western-blot experiments. Pharmacological inhibitors, siRNA, CrispR-Cas9 will be used to interfere with candidate gene/pathways and validate their functional involvement. PDX cells from different resistant patients that are maintained in co-culture conditions on top of mesenchymal feeder cells will be used to screen drug libraries to identify leukemic dependencies that can become new targets.
Candidate:
We are looking for a motivated and enthusiastic Master student with experience in classical cell biology, biochemistry, molecular biology techniques, applied to cancer projects. Experience with flow cytometry and mouse handling could be a plus. The ideal candidate likes to work within a team with a strong desire to develop his/her autonomy.
Overall, this PhD project has the potential to identify new therapeutic strategies to treat relapsed pediatric T-ALL, which could have a significant impact on improving the survival rate of children affected by this disease.
Bibliography:
Can one target T-cell ALL
Adolfo Ferrando
Best Practice & Research Clinical Haematology 31 (2018) 361–366
https://doi.org/10.1016/j.beha.2018.10.001
Contact:
Dr Jean-François Peyron, directeur de recherche Inserm
Jean-Francois.Peyron@unice.fr
Team Fundamental to Translational Research on Dysregulated Hematopoiesis
Cytoplasmic Iron-sulfur Assembly as a key player in hematopoiesis
Fe-S clusters are molecular complexes of iron bound to sulfur that are essential for enzymatic activities, redox metabolism, and regulation of gene expression. Most Fe/S proteins contain either a [2Fe–2S] cluster or a cubic [4Fe–4S] cluster. In eukaryotes, up to 1% of proteins bind Fe-S clusters, including key enzymes in DNA replication and repair, protein translation, and energy metabolism (1).
Two distinct machinery complexes drive Fe-S cluster inclusion into proteins. First, the mitochondrial ISC machinery. These enzymes generate Fe-S for use in mitochondrial proteins. Second, the cytoplasmic iron sulfur assembly (CIA) targeting complex playing a key role in Fe-S transfer into cytoplasmic and nuclear proteins.
Sideroblastic anemias (SA) are mainly acquired bone marrow diseases with abnormal erythroid precursors called ring sideroblasts containing deposits of non-heme iron in mitochondria. Hereditary SA is caused by mutations in genes that are involved in heme synthesis, iron-sulfur cluster biogenesis, or mitochondrial metabolism.
Human diseases caused by hereditary Fe-S biogenesis defect have been discovered, most of them involving the ISC machinery. They originate mainly from hypomorphic mutations, leading in most cases to neuromuscular affections like Friedreich Ataxia (Fxn) and hereditary myopathies (ISCU, FDX1L, NUBPL…). Regarding hereditary SA, the most common is caused by mutation of the X-located gene coding for the ALAS2 enzyme. ALAS2, GLRX5, ABCB7 gene mutations cause microcytic anemia while SLC19A2, PUS1, YARS2, TRNT1 mitochondrial gene mutations cause macrocytic anemia.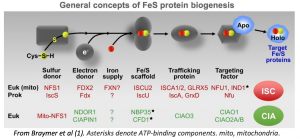
To date, no inherited disease has been described involving the proteins forming the CIA complex of Fe-S transport and assembly in the cytoplasm, except for one case that we discuss thereafter.
Case Report:
The Pediatrics Department of the Nice CHU has been treating in the last 18 months a female patient with a severe sideroblastic anemia discovered at the age of 18y. Sideroblastic anemias (SA) are mainly acquired bone marrow diseases with abnormal erythroid precursors called ring sideroblasts in mitochondria. Hereditary SA is caused by mutations in genes that are involved in heme synthesis, iron-sulfur cluster biogenesis, or mitochondrial metabolism. Only discrete immunologic alterations could be evidenced and no associated organ dysfunctions were found in our patient. The anemia was accompanied by a marked iron excess in blood and organs, preceding transfusional overload.
We have thus called this late onset disease: Isolated Sideroblastic Anemia of Congenital origine (ISAC). The results a whole exome sequencing evidenced a compound heterozygous mutation affecting a protein of the CIA complex, one allele displaying a mutation leading to early protein truncation while in the other an ambivalent amino-acid was replaced by a positively charged one. The inherited character was confirmed by the gene sequencing in both parents, each of them carrying one mutation. This amino-acid substitution is located near one Fe-S cluster of the protein, thus likely leading to functional alteration, and or protein. The deletion of CIA complex members in mouse is lethal at the embryonic level, highlighting the essential role of this Fe-S pathway. Thus, we hypothezised that in our patient, the missense mutation could affect severely, but not completely, the level and function of the protein. The fact that the patient’s cells growth was largely affected in culture led us to hypothezise that CIA function is critical for cell homeostatis and resistance to oxydative stress. Given the role of hypoxia in cancer cells and the importance of iron-sulfur metabolism in cancer, and notably in leukemia, we have noticed that severeal proteins of CIA are overexpressed in acute myeloid leukemia (AML) and also in representative cell lines of childhood ALL. Several proteins contain iron-sulfur groups fulfilling essential functions in cell cycle control, genome maintenance and stability, which are important to leukemic cells. The CIA pathway may be critical in cell survival as several DNA metabolic enzymes are dependent on complete [4Fe-4S] clusters for activity. Enzymes involved in DNA replication, DNA synthesis, and DNA repair, including polymerases, helicases like RTEL1 or RAD3/XPD, nucleases, demethylases, ROS1 for instance, and ribonucleotide reductases, require Fe–S biogenesis in order to fulfill their functions. Some proteins like CISD1/2 at the beginning of the CIA pathway are thought to be novel cancer targets
Objectives:
- to study the mechanisms leading to sideroblastic anemia in our patient
- to investigate potential therapies allowing to circumvent the CIA functional defect, thus allowing to overcome the sideroblastic blockade
- to study the precise role of CIA in the maintenance of long term erythropoiesis
- to elucidate the dependency on CIA in leukemic cell lines and the efficacy of CIA blockade, either alone or in association with known antileukemic compounds
Bibliography: Braymer JJ, Freibert SA, Rakwalska-Bange M, Lill R. Mechanistic concepts of iron-sulfur protein biogenesis in Biology. Biochim Biophys Acta Mol Cell Res. 2021 Jan;1868(1):118863. doi: 10.1016/j.bbamcr.2020.118863. Epub 2020 Sep 30. PMID: 33007329.
Informations:
The project is led by Pierre Rohrlich within the DYSHEMA team led by Jean-François Peyron at the Centre Méditerranéen de Médecine Moléculaire. The coordinator has more than 30 years of experience in hematology of children and young adults, he is in charge of the Competence Center for Constitutional Diseases of the Red Blood Cell and Erythropoiesis (MCGRE) at the University Hospital of Nice, and works in collaboration with the expert centers (Marseille, Mondor, Bichat) His clinical experience is completed by a scientific expertise acquired at the Pasteur Institute where he studied HFE, a molecule involved in martial metabolism.
The consortium brings together three research teams, two at C3M: the DYSHEMA team led by Jean-François Peyron and Pierre Rohrlich, and the MCARE team led by Els Verhoeyen, and a Parisian team at Bichat: Prof. Katell Peoch and Prof. Kannengiesser (Centre de Recherche sur l’Inflammation UMRs INSERM 1149).
Contact:
Dr Jean-François Peyron, directeur de recherche Inserm
Jean-Francois.Peyron@unice.fr
Team Microenvironment, Signaling and Cancer
Role of calcium channels in melanoma biomechanical reprogramming and therapeutic resistance
Melanoma is a skin cancer that is particularly difficult to cure in the advanced stages of the disease because of its aggressiveness, plasticity and therapeutic resistance. The treatment of metastatic melanoma has undergone a revolution with the arrival of targeted therapies and immunotherapies. However, these therapies only benefit few patient and their use is limited in time due to the rapid emergence of resistance. Our team is interested in the mechanisms that account for melanoma resistance to anti-cancer therapies with the aim of identifying new therapeutic targets to improve the efficacy of existing treatments and to prolong their effects. Our recent results establish a close relationship between tumour cell plasticity, their sensitivity to mechanical signals from the microenvironment and therapeutic resistance. We have shown that the acquisition of a dedifferentiated phenotype is associated with a biomechanical reprogramming of the melanoma cells that confers them increased sensitivity to changes in extracellular matrix stiffness. This biomechanical reprogramming is associated with changes in the expression of several calcium channels. As these channels are described as sensors of the physico-chemical properties of the tumour microenvironment, our hypothesis is that they may contribute to the mechanosensitivity of melanoma cells and may influence their proliferation, motility and therapeutic response.
To test these hypotheses, the successful candidate will combine molecular and cellular biology as well as biochemical and biophysical approaches using relevant in vitro and in vivo preclinical models.
The thesis project will be based on 3 axes :
- Influence of changes in the rigidity of the extracellular matrix and the actors of mechanotransduction on the expression/activation of calcium channels and characterisation of the mechanisms involved.
- Role of calcium channels in the proliferation, invasion and resistance of melanoma in vitro
- Impact of pharmacological targeting of calcium channels on the progression and therapeutic resistance of melanoma in vivo.
This work will improve our knowledge of the dialogue between tumour cells and the matrix network and will provide a better understanding of the mechanisms involved in therapeutic resistance. In addition, it should lead to the identification of new therapeutic targets and will open up innovative avenues for improving the management of refractory metastatic melanoma.
Keywords: Melanoma, Microenvironment, Mechanotransduction, calcium channels, Cell signaling, therapeutic resistance.
Candidate:
Biomedical PhD student :
- Master’s degree (or equivalent) in life science, cell biology, cancer, or any related area.
- Thorough knowledge of and inherent interest in subjects such as Cancer Research, Molecular Biomedicine, molecular mechanisms of tumor initiation and progression, mechanotransduction and Ion channel biology.
- Solid bench expertise in cell culture and excellent skills (with track record) in basic lab experimentation (Cell culture, Western-blot, qPCR…) are required; Hand-on experience in electrophysiology would be an asset and candidate holding an accreditation for animal experimentation will be preferred.
- Enthusiastic and motivated team player who enjoys bench work and has a can-do attitude, innovative thinking, demonstrated organizational skills, a keen sense of responsibility. The candidate is expected to be highly rigorous, reliable, and easily adapt to different working environments.
- Inclined towards multidisciplinary approaches (biology, biophysics, bioinformatic, etc.) and able and willing to interact with different interlocutors.
Bibliography:
Shoji KF,…, Tartare-Deckert S, Penna A. The mechanosensitive TRPV2 calcium channel promotes human melanoma invasiveness and metastatic potential. EMBO Rep, e55069, 2023.
Diazzi S,…, Girard C.A,…, Mari B*, Tartare-Deckert S*. Blockade of pro-fibrotic response mediated by the miR-143/-145 cluster prevents targeted therapy-induced phenotypic plasticity and resistance in melanoma. EMBO Mol Med, 4, e15295, 2022.
Berestjuk I,…, Girard CA, Deckert M*, Tartare-Deckert S*. « Targeting Discoidin Domain Receptors DDR1 and DDR2 overcomes matrix-mediated tumor cell adaptation and tolerance to BRAF-targeted therapy in melanoma. EMBO Mol Med, 14, e11814,2022.
Girard CA*,…, Deckert M*, Tartare-Deckert S*. A feed-forward mechanosignaling loop confers resistance to therapies targeting the MAPK pathway in BRAF-mutant melanoma. Cancer Res, 80, 1927-1941, 2020.
Leverrier-Penna S,…, Penna A. Insights and perspectives on calcium channel functions in the cockpit of cancerous space invaders. Cell Calcium, 90:102251, 2020.
Lecacheur M, Girard CA,…, Tartare-Deckert S. Echappement thérapeutique du mélanome : la piste biomécanique. Med Sci(Paris), 36, 961-965, 2020.
Contacts:
PhD supervisor :
Dr Christophe GIRARD
christophe.girard@univ-cotedazur.fr
Phone : +33 4 89 15 38 52
PhD co-supervisor :
Dr Aubin PENNA
aubin.penna@univ-poitiers.fr
Phone : +33 5 49 45 39 96
Interested candidates should send a CV and a cover letter to christophe.girard@univ-cotedazur.fr








