The aims of the present team (created in 2008), composed of clinicians and basic scientists, are to better understand the hepatic complications associated with obesity (metabolic dysfunction-associated steatotic liver disease: MASLD) and with chronic alcohol consumption (alcohol related liver disease, ALD). These chronic liver diseases range from steatosis to steatohepatitis (metabolic dysfunction and alcohol related steatohepatitis, MASH and ASH), fibrosis, cirrhosis and finally hepatocellular carcinoma. MASLD and ALD are the main causes of cirrhosis and increase the risk of liver-related death with limited therapeutic options available. It is urgent to better diagnosis and treat these chronic liver diseases. Our translational researches mainly focus on 1) the identification of new markers/actors of the progression of these chronic liver diseases (MASLD/ALD). We take advantage of our cohorts of obese and alcoholic patients; 2) the study of potential players in the progression of MASLD and ALD including the cell matrix interaction- (CD44), non-receptor tyrosine kinase- and innate lymphoid cell- dependent pathways as well as metabolic pathways regulating immune functions; 3) the impact of targeting these pathways is investigated by preclinical approaches. Over the past few years, the team have made significant contributions to provide new insights into the understanding of these chronic liver diseases in order to propose a better diagnostic and new therapeutic targets.

Our translational research mainly focuses on:
- the identification of new markers/actors of the progression of these chronic liver diseases (MASLD/ALD). We draw on our cohorts of obese and alcoholic patients;
- the study of potential players in the progression of MASLD and ALD including pathways dependent on the cell-matrix interaction (CD44), non-receptor tyrosine kinases and innate lymphoid cells pathways as well as metabolic pathways regulating immune functions;
- the impact of targeting these pathways is investigated by preclinical approaches. Over the past few years, the team has made significant contributions to provide new insights into the understanding of these chronic liver diseases in order to propose better diagnostics and new therapeutic targets.

Projects

R. ANTYUniversity Professor - Hospital Practitioner (PU-PH)
Mail anty.r@chu-nice.fr

P. GUALResearch Director
Mail philippe.gual@inserm.fr
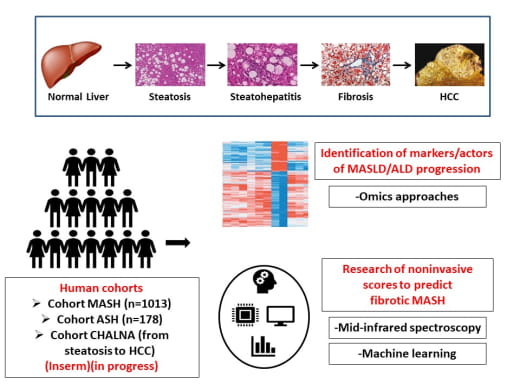
To predict MASH and fibrotic MASH, there is an urgent need for noninvasive scores that can be easily measured. In addition, identification of markers/actors of MASLD and ALD progression will provide a better understanding of the pathogenesis of these chronic liver diseases.
To this aim, we mainly focus on:
- the establishment of a cohort of well-characterized patients with ALD and MASLD. We have already established cohorts of morbidly obese patients and alcoholic patients with biopsy-proven MASLD/ALD, complete biological and clinical data and blood, DNA and liver samples. Patients with ALD or MASLD from hepatic steatosis to HCC are currently included in a new cohort.
- the search for actors involved in the progression of these chronic liver diseases through Omics studies. Only a group of miRNA/genes/pathways with modified expression levels in patients with steatohepatitis will be further studied.
- the search for noninvasive scores to predict fibrotic MASH. We mainly develop new scores based on simple clinical and biological criteria using an artificial intelligence approach. Identification of new markers via our omics research will feed our databases. External validation of the proposed models will be performed by our national and international partners.

A. IANNELLIUniversity Professor - Hospital Practitioner (PU-PH)
Mail IANNELLI.A@chu-nice.fr

S. PATOURAUXHospital practitioner (PH)
Mail patouraux.s@chu-nice.fr

A. TRANUniversity Professor - Hospital Practitioner (PU-PH)
Mail tran.a@chu-nice.fr
S. BONNAFOUSResearch engineer
Mail sbonnafo@univ-cotedazur.fr

G. FavreUniversity Professor - Hospital Practitioner (PU-PH)
Mail favre.g@chu-nice.fr

P. GUALResearch Director
Mail philippe.gual@inserm.fr
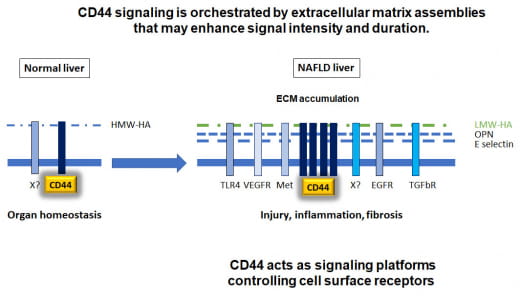
In normal conditions high molecular weight hyaluronic acid (HW-HA) is a major receptor for CD44. In MASLD, CD44 itself and many of its interactors, either located in the ECM or within the cytoplasmic membrane are overexpressed. High expression of these molecules is found in MASLD. We propose that CD44 acts as a central integrator and amplifier by transmitting signals from the ECM into the cytoplasm thus triggering pro-inflammatory signaling that drives MASLD. Low/High molecular weight HA; X, unknown molecular interactors to be identified here.
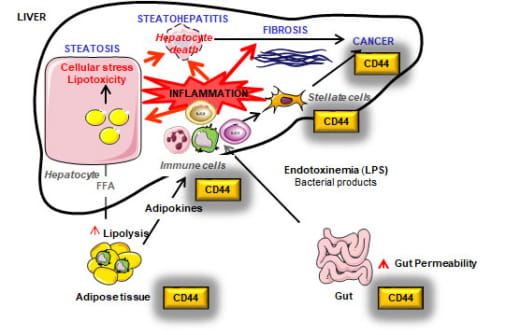
Chronic inflammation and the associated fibrosis are the progressive forms of MASLD/ALD and no pharmacological treatment is yet available. We investigate how the extracellular matrix and its receptors play a key role in orchestrating the chronic inflammation and fibrosis that contribute to MASLD/ALD progression.
We mainly focus on osteopontin, hyaluronan, and CD44, which are co-overexpressed in human and mouse MASLD/ALD and are part of a common network. A better understanding of their combined role in liver inflammation may provide novel tools for diagnosis and for therapeutic strategies. This project is conducted via multidisciplinary approaches combining omics, transgenic mouse models, novel therapeutic approaches targeting CD44 and a large cohort of human samples to gain a comprehensive understanding of MASLD/ALD progression.

F. SOYSOUVANHPost-doctoral fellow
Mail frederic.soysouvanh@univ-cotedazur.fr

D. ROUSSEAUResearch engineer
Mail droussea@univ-cotedazur.fr

S. BONNAFOUSResearch engineer
Mail sbonnafo@univ-cotedazur.fr

S. PATOURAUXHospital practitioner (PH)
Mail patouraux.s@chu-nice.fr

R. ANTYUniversity Professor - Hospital Practitioner (PU-PH)
Mail anty.r@chu-nice.fr

A. TRANUniversity Professor - Hospital Practitioner (PU-PH)
Mail tran.a@chu-nice.fr

C. LUCIResearcher
Mail carmelo.luci@inserm.fr
J. FREREPhD student
Mail julien.frere@etu.univ-cotedazur.fr

A. StrazzullaPhD student
Mail axelle.strazzulla@etu.univ-cotedazur.fr
A. BelmerLecturer
Mail arnauld.belmer@gmail.com

C. LUCIResearcher
Mail carmelo.luci@inserm.fr
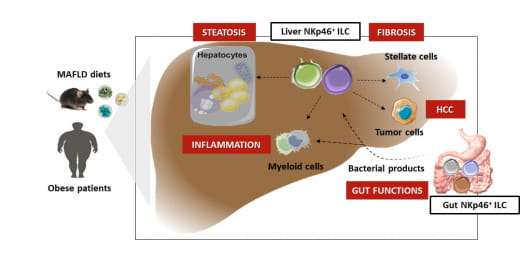
Chronic low-grade inflammation associated with obesity plays a key role in the development and progression of MASLD (Luci et al., 2020). A better understanding of the regulation of inflammation mechanisms and functions of resident and/or recruited immune cells into the liver would allow new diagnostic and therapeutic approaches against MASLD to be proposed.
In this context, our work focuses on the contribution of innate lymphoid cells (ILC) in the development of MASLD. Specifically, we focus on natural killer (NK) cells and type 1 helper ILCs, two key players in the regulation of inflammatory and metabolic responses (Luci et al., 2009, Vivier et al., 2018, Luci et al., 2021). Moreover, it is now clearly accepted that the functions, survival and differentiation of these cells are finely controlled by metabolic pathways (glycolysis, oxidative phosphorylation, etc.) (Makowski et al., 2020). Given the growing interest in the role of NK cells in metabolic disorders and particularly in MASLD (Luci et al., 2019; Bourinet at al., 2023), understanding their metabolism in order to better exploit their effector functions is of fundamental and translational interest to open innovative and promising therapeutic perspectives for MASLD.

D. ROUSSEAUResearch engineer
Mail droussea@univ-cotedazur.fr

S. BONNAFOUSResearch engineer
Mail sbonnafo@univ-cotedazur.fr

S. PATOURAUXHospital practitioner (PH)
Mail patouraux.s@chu-nice.fr

R. ANTYUniversity Professor - Hospital Practitioner (PU-PH)
Mail anty.r@chu-nice.fr

A. TRANUniversity Professor - Hospital Practitioner (PU-PH)
Mail tran.a@chu-nice.fr

P. GUALResearch Director
Mail philippe.gual@inserm.fr

P. GUALResearch Director
Mail philippe.gual@inserm.fr

C. LUCIResearcher
Mail carmelo.luci@inserm.fr
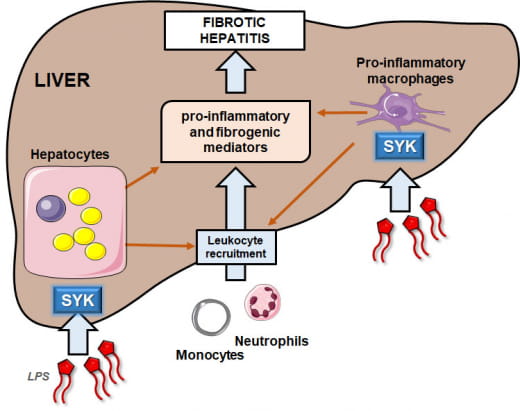
Activation of innate immune cells by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) via Toll-like receptor (TLR) signaling is a key mechanism in the pathogenesis of chronic inflammatory diseases. Spleen tyrosine kinase (SYK) emerges as a novel regulator of the TLR pathway.
We recently highlighted that the SYK-3BP2 pathway, by regulating LPS-TLR4 responses in hepatocytes and liver macrophages, contributes to the onset of NASH. The hepatic expression of SYK is also upregulated with MASH and correlates with liver macrophages in biopsy-proven MASLD patients (Cell Mol Gastroenterol Hepatol. 2022;13(1):173-191). Since SYK also regulates hepatic stellate cell activation, liver fibrosis and MASLD progression to HCC by regulating myeloid cells, our next goals will be:
- to better decipher SYK-dependent signaling pathways regulating macrophage functions in our models of chronic inflammation.
- to preclinically evaluate how targeting of SYK in specific liver cells and by pharmacologic inhibitors improves the pathogenic chronic inflammation in mouse models of fibrotic MASH and MASH-driven HCC. This project is conducted in collaboration with Marcel Deckert and via multidisciplinary approaches combining omics and novel therapeutic approaches targeting SYK (new inhibitors and specific targeting of liver cells)

F. SOYSOUVANHPost-doctoral fellow
Mail frederic.soysouvanh@univ-cotedazur.fr

D. ROUSSEAUResearch engineer
Mail droussea@univ-cotedazur.fr

S. BONNAFOUSResearch engineer
Mail sbonnafo@univ-cotedazur.fr

S. PATOURAUXHospital practitioner (PH)
Mail patouraux.s@chu-nice.fr

M. DeckertResearch Director
Mail marcel.deckert@univ-cotedazur.fr
Publications
Bax Inhibitor-1 preserves pancreatic β-cell proteostasis by limiting proinsulin misfolding and programmed cell death.Authors Blanc M, Habbouche L, Xiao P, Lebeaupin C, Janona M, Vaillant N, Irondelle M, Gilleron J, Murcy F, Rousseau D, Luci C, Barouillet T, Marchetti S, Lacas-Gervais S, Yvan-Charvet L, Gual P, Cardozo AK, Bailly-Maitre B
Cell death & disease May 2024
Enhanced liver fibrosis score is stable after withdrawal in patients with heavy alcohol consumption: A pilot study.Authors Lévi-Strauss T, Gal J, Gelsi E, Truchi R, Ouizeman DJ, Hinault C, Chinetti G, Tran A, Gual P, Anty R
Alcohol, clinical & experimental research Apr 2024
Roles of innate lymphoid cells in metabolic and alcohol-associated liver diseases.Authors Bourinet M, Anty R, Gual P, Luci C
JHEP reports : innovation in hepatology Feb 2024
Show all publicationsPatents
Co-inventors B. Bailly-Maitre, P. GUAL, A. TRAN
Co-inventors B. Bailly-Maitre, P. GUAL, A. TRAN
Co-inventors C. LUCI, ANJUERE Fabienne, HACINI-RACHINEL Feriel, VIAL Emmanuel


