Prostate cancer is the most common cancer in men and is the third leading cause of cancer deaths. The major problems with this cancer are the absence of a marker of aggressiveness and resistance to treatment. Indeed, there is currently no marker to predict the aggressiveness of this cancer, which kills more than 8000 people per year in France. Our laboratory is interested in studying the molecular and cellular mechanisms involved in the formation of metastases and resistance to treatments.
The fate of tumor cells depends on their microenvironment and the exposure to environmental factors. More generally, cancer cells adapt to their complex ecosystem to sustain proliferation and dissemination. This adaptation requires the regulation of metabolic pathways that promote cell survival, motility, invasiveness and tumor angiogenesis. These capacities involve active communications between tumor cells and their environment regulated by physicochemical variables: nutrients, oxygen concentration (Hypoxia), hormones, and pH. Our team project encompasses the study of cancer cell metabolism as well as interference of endocrine disruptors on cancer progression.

Projects

F. BOSTResearch Director
Mail frederic.bost@univ-cotedazur.fr
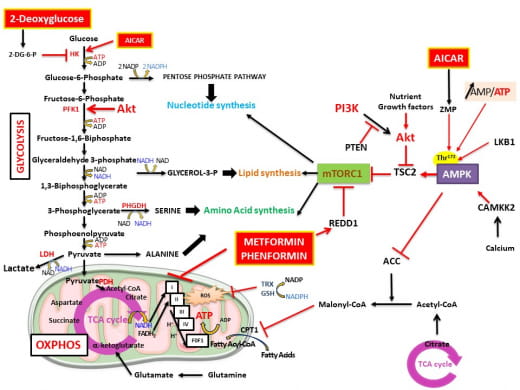
Cancer cells have a different metabolism than normal cells. They reprogram their metabolism to meet bioenergetic and biosynthetic demands in order to proliferate and invade surrounding tissues. Several studies have shown that metabolic disruptors can slow down tumor growth. Our team was one of the first to demonstrate that metformin, a drug initially prescribed for type 2 diabetes, targets mitochondrial metabolism and induces energy stress in prostate cancer cells. We have shown that metformin slows tumor growth and significantly reduces metastasis. More recently, we have shown that PGC-1 alpha, a transcription factor coactivator, regulates polyamine synthesis and tumor cell aggressiveness. Our research project is to better understand how, by interfering with cellular metabolism, we can target the prostate cancer cell to identify new therapeutic targets.

F. BOSTResearch Director
Mail frederic.bost@univ-cotedazur.fr

P. PeraldiResearcher
Mail Pascal.Peraldi@unice.fr

M. KahiPhD student
Mail Michel.KAHI@univ-cotedazur.fr

A. MazzuResearch engineer
Mail Abigail.Mazzu@unice.fr
JM. FerreroUniversity Professor - Hospital Practitioner (PU-PH)
Mail jean-marc.ferrero@nice.unicancer.fr

M. Pujalte-MartinPhD student
Mail Marc.PUJALTE-MARTIN@nice.unicancer.fr

N. MazureResearch Director
Mail nathalie.mazure@univ-cotedazur.fr
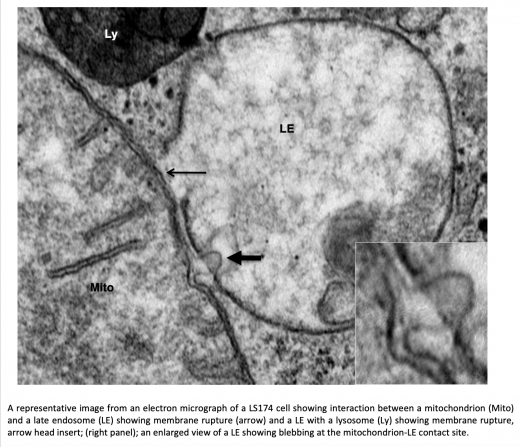
Our team has identified a novel and compelling hypoxia-inducible factor (HIF)- and TP53/TP73-dependent molecular mechanism that involves direct contact between mitochondria and endolysosomes in cancer cells (breast, colon, kidney and lung cancers). This cross-contact involves enlarged mitochondria, exhibiting mitochondrial cristae remodeling, and truncation of the C-terminal voltage-dependent mitochondrial anion channel (VDAC1 to VDAC1-ΔC) by lysosomal peptidases (Legumain-LGM) in hypoxia. This results in increased metabolic regulation of both oxidative phosphorylation (OXPHOS) and glycolysis and increased resistance to chemotherapy. With its three isoforms, VDAC1, 2, and 3, the channel family constitutes the most abundant pore-forming proteins in the mitochondrial outer membrane (MOM) where they control the flow of ADP, ATP, ions, respiratory substrates, and metabolites through the organelle. By binding to VDAC, several cytoplasmic and cytoskeletal proteins, including tubulin, modulate MOM permeability and thus control a wide variety of mitochondrial functions and probably the response to chemotherapy. Aberrant VDAC expression or function has been reported in many tumors, indicating that targeting VDAC is of therapeutic importance for cancer treatment.
We have shown that the hypoxia-induced cleaved form of VDAC1 (i.e., VDAC1-ΔC) is a critical regulator of glycolysis and ciliogenesis, as the conversion of VDAC1 to VDAC1-ΔC correlates with the loss of primary cilium (PC) and increased glycolysis and mitochondrial respiration. Our studies demonstrate that VDAC1-ΔC reprograms PC-deficient cells to utilize more metabolites (i.e. lactate, mannitol, fructose, etc.) promoting cell growth in a hypoxic microenvironment.

N. MazureResearch Director
Mail nathalie.mazure@univ-cotedazur.fr

Y. GuoPhD student
Mail yingbo.guo@etu.univ-cotedazur.fr

S. PengPhD student
Mail siyong.peng@etu.univ-evry.fr

A. MazzuResearch engineer
Mail Abigail.Mazzu@unice.fr

C. HinaultUniversity Lecturer - Hospital practitioner (MCU-PH)
Mail Charlotte.Hinault@unice.fr

N. ChevalierUniversity Professor - Hospital Practitioner (PU-PH)
Mail Nicolas.Chevalier@unice.f
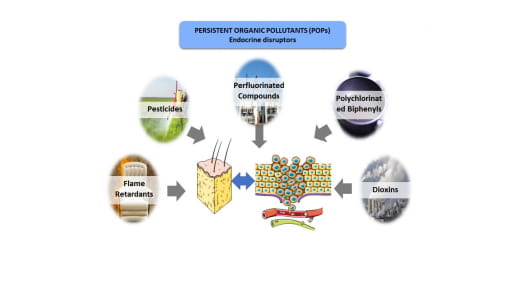
Endocrine-disrupting chemicals (EDCs) are a heterogeneous category of environmental pollutants mainly produced by the chemical industry: pesticides, plastics, pharmaceuticals, paints, glues, etc. Among EDCs, persistent organic pollutants (POPs) are a major source of concern for human health; they are persistent in the environment for several decades and, due to their lipophilic nature, they are bioaccumulative in organisms, particularly in adipose tissue (AT). Although the AT initially plays a protective role by storing these EDCs, the EDCs can cause a modification of the adipose secretome, and be released in the body, especially after weight loss. These changes can have a negative impact on the body, and on the development of certain hormone-sensitive cancers, well beyond the initial exposure period. Our overall objective is to characterize the modifications induced by EDCs on the adipose secretome, and to determine their impact on the proliferation of prostate cells. POPs are grouped into five categories: polychlorinated dibenzoparadioxins (PCDDs) and polychlorinated dibenzofurans (PCBFs), polychlorinated biphenyls (PCBs), organochlorine pesticides (OCs) (which have been banned since 1970 because of their toxicity), polybrominated flame retardants (PBDEs) and perfluorooctane derivatives (PFOS and PFOAs) found in non-stick coatings. As some of these POPs have a hormonally-mimetic activity, the IARC (International Agency for Research on Cancer) has suggested a very probable role of exposure to these POPs in the occurrence and development of hormone-sensitive cancers: thyroid, prostate, testis, breast, and ovary. However, the exact risk of POP exposure remains difficult to estimate and quantify.

C. HinaultUniversity Lecturer - Hospital practitioner (MCU-PH)
Mail Charlotte.Hinault@unice.fr

N. ChevalierUniversity Professor - Hospital Practitioner (PU-PH)
Mail Nicolas.Chevalier@unice.f

F. BOSTResearch Director
Mail frederic.bost@univ-cotedazur.fr
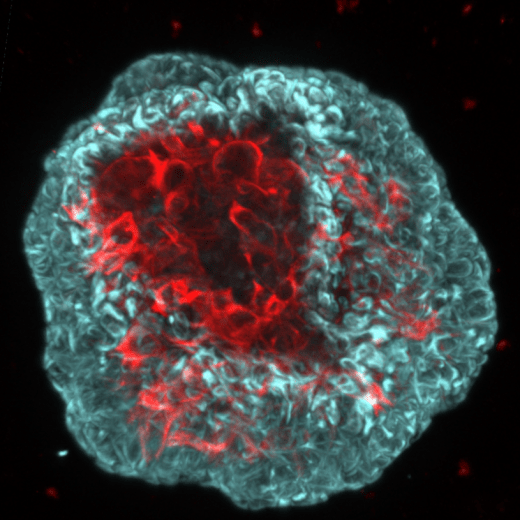
The vast majority of "anti-cancer" molecules validated in the laboratory prove ineffective in clinical trials. This dramatic observation is due to the lack of relevance of the experimental models used in research (mice and cell lines). For example, genetically modified mouse models do not reflect the histological complexity and genetic heterogeneity of human cancers. The spectacular development of 3D cell culture technologies and the creation, from progenitor cells or human "tumor stem cells," of self-organizing organotypic structures such as organoids/tumoroids, is revolutionizing translational medicine. The goal of "precision medicine" is to deliver each patient a specific treatment that can be tested "a priori" on tumor cells derived from their own tumor. In this context, we have developed in the laboratory the production and culture of organoids/tumoroids derived from cells of prostate cancer patients. This strategy allows us to obtain, for each patient, populations of organoids (healthy areas) and tumoroids (cancerous areas) from fresh surgical specimens.

J. MurdacaResearch engineer
Mail Joseph.MURDACA@univ-cotedazur.fr

7th Yamada Symposium
From 25 to 30 Août 2024 - Kobe (Japon)
https://smartconf.jp/content/icbrp2024/

From 22 to 24 november 2023 - Nice (France)
https://www.metabolism-cancer.com/

From 16th to 18th December 2024 - Le Croisic (France)

Metabolic and Immunosuppressive ASPECTS of prostate cancer
20 November 2024 - Palais des Congrès de Paris - 2 place de la Porte Maillot - 75017 Paris
https://www.c3m-nice.fr/admin-thuria/?loggedout=true&wp_lang=fr_FR
Publications
YAP1 modulation of primary cilia-mediated ciliogenesis in 2D and 3D prostate cancer models.Authors Guo Y, Dupart M, Irondelle M, Peraldi P, Bost F, Mazure NM
FEBS letters Oct 2024
Publisher Correction: LKB1-SIK2 loss drives uveal melanoma proliferation and hypersensitivity to SLC8A1 and ROS inhibition.Authors Proteau S, Krossa I, Husser C, Guéguinou M, Sella F, Bille K, Irondelle M, Dalmasso M, Barouillet T, Cheli Y, Pisibon C, Arrighi N, Nahon-Estève S, Martel A, Gastaud L, Lassalle S, Mignen O, Brest P, Mazure NM, Bost F, Baillif S, Landreville S, Turcotte S, Hasson D, Carcamo S, Vandier C, Bernstein E, Yvan-Charvet L, Levesque MP, Ballotti R, Bertolotto C, Strub T
EMBO molecular medicine Aug 2024
Vitiligo auto-immune response upon oxidative stress-related mitochondrial DNA release opens up new therapeutic strategies.Authors Sant'Anna-Silva ACB, Botton T, Rossi A, Dobner J, Bzioueche H, Thach N, Blot L, Pagnotta S, Kleszczynski K, Steinbrink K, Mazure NM, Rocchi S, Krutmann J, Passeron T, Tulic MK
Clinical and translational medicine Aug 2024
Show all publicationsPatents
Co-inventors N. Mazure, Lucilla Fabbri Maeva Dufies
Co-inventors N. Mazure, Lucilla Fabbri Maeva Dufies






